ABSD
ABSD (Acinetobacter baumannii K-antigen three-dimensional Structure Database) is a 3D structural repository of A. baumannii 64 K-antigen monomers (K1 to K30 (except K9, K10, K18, K23, K28, K29), K32 to K49 (except K34, K36, K38, K40, K41), K53 to K57 (except K56), K73, K74, K82 to K93 (except K84), K98, K106, K112, K116, K125, K127, K128, K139, K144 and K218). The sugar composition, linkages, substitutions and presence of special sugars are determined using chemical analysis, Smith degradation, methylation analysis, NMR measurements etc. For details, visit the documentation page.
Acinetobacter baumannii
The greatest challenges faced by human health in recent times are the development of diseases associated with antibiotic-resistant bacteria. The most widely spread MDR pathogen in recent times is Acinetobacter baumannii. They belong to a certain group of pathogens called ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter. baumannii, Pseudomonas aeruginosa, and Enterobacter spp) pathogens, as they can easily escape antibiotic treatment. Acinetobacter baumannii exhibits various patterns of resistance such as Multi-drug resistance(MDR-resistance to atleast three classes of drugs), Extensive-drug resistance(XDR-MDR + resistance to carbapenems) and Pan-drug resistance(XDR + resistance to polymyxins). Various virulence factors that help the organism to acquire resistance are surface glycoconjugates such as capsular polysaccharide(CPS), lipooligosaccharide(LOS), poly-beta-(1-6)N-acetylglucosamine(PNAG), type IV pili, Csu pili. The capsular polysaccharide (CPS) is an important virulence factor. A total of 92 K antigen types have been identified through serological reactivity tests, molecular genotyping and phage typing.
|
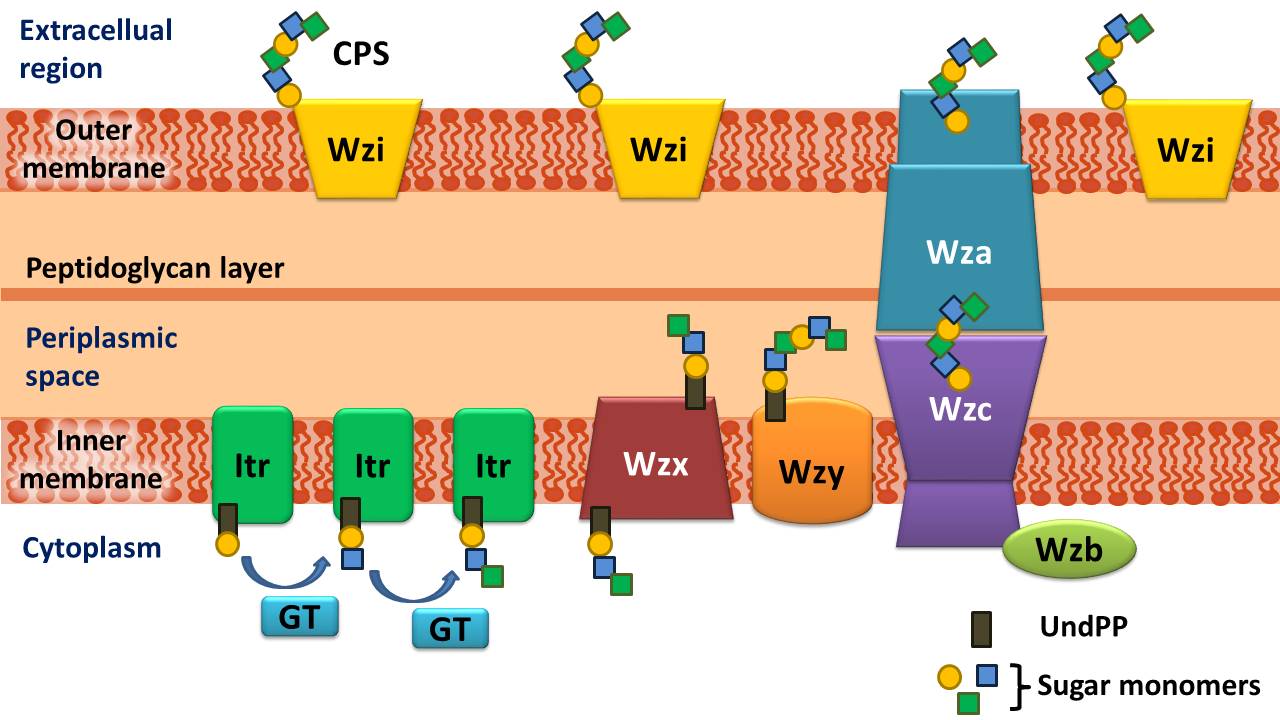
Schematic representation of proteins involved in the biosynthesis and export of K-antigens
|
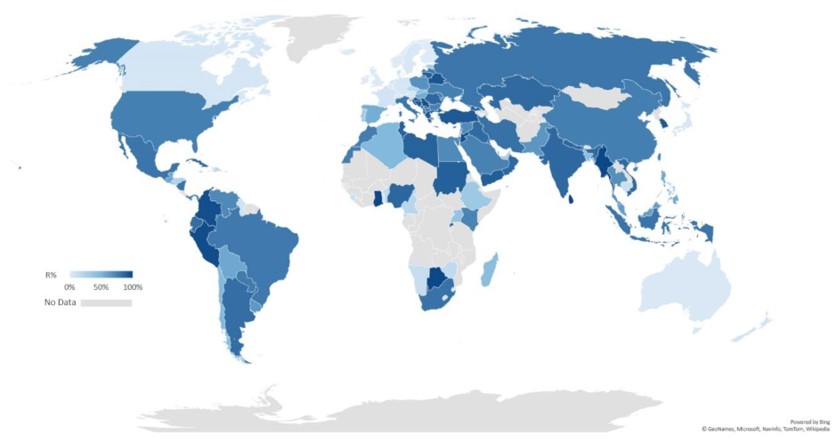
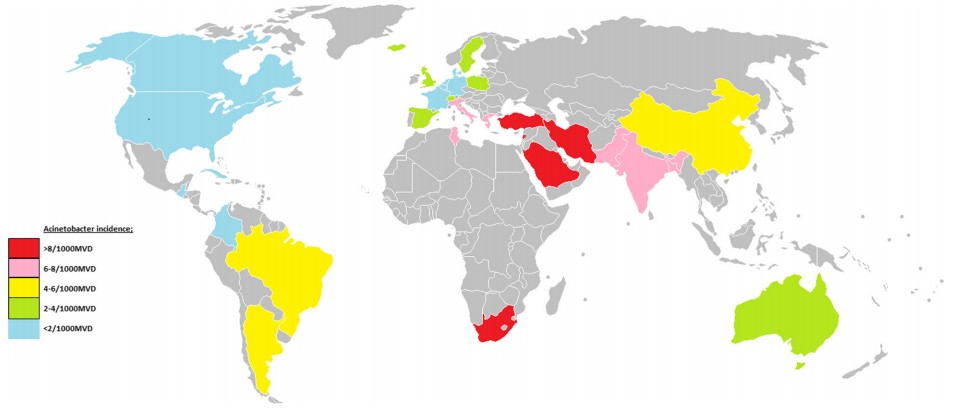
Recent updates on antimicrobial resistance [Note: Click on the picture to go to the source page.] |
Capsule polysaccharide assembly and export
CPS or K-antigens are one of the major virulence determinants in Gram-negative bacteria such as Acinetobacter baumannii. CPS forms a discrete layer on the bacterial surface, providing protection from diverse environmental conditions, assisting in evasion of host immune defenses, and increasing resistance to a number of antimicrobial compounds. In A. baumannii, capsule assembly and export occurs via a Wzx/Wzy-dependent pathway. Typically consisting of 4 to 6 sugars, the K unit is assembled on the lipid carrier molecule undecaprenyl pyrophosphate (Und-P), which provides a scaffold for the growing sugar chain. The first sugar in the K unit is recruited by an inner membrane (IM)-bound initial transferase (Itr), followed by the sequential addition of sugars to the growing K unit by specific glycosyl transferase (Gtr) enzymes. Each K unit is then transferred to the periplasmic side of the IM by the Wzx translocase and polymerized by Wzy, which transfers the growing polysaccharide chain from one Und-P carrier to the next incoming subunit. After the CPS polymer is synthesized, it is transported to the cell surface via a highly co-ordinated process involving the interaction of three proteins; Wza, Wzb, and Wzc, that comprise the export machinery.
Carbapenem resistance
A. baumannii strains are currently being isolated in clinics making treatment increasingly difficult. In particular, a 'quantum leap' in the difficulty to treat A. baumannii infections occurred with the appearance of CRAB(Carbapenem Resistant Acinetobacter baumannii) strains. One of the most common mechanisms of resistance to β-lactam antibiotics is their enzymatic hydrolysis mediated by β-lactamases. There are 4 different types of beta-lactamases proposed by Ambler:A,B,C and D. Enzymes belonging to classes A, C, and D catalyze the hydrolysis of the β-lactam substrate forming an intermediate covalent acyl-enzyme complex with a serine residue within the active site. In the case of class B β-lactamases, the hydrolysis reaction is mediated by one or more zinc ions. OXA β-lactamases are class D enzymes that were originally differentiated by TEM enzymes by their ability to hydrolyze oxacillin. OXA enzymes were first isolated from Scotland and later found in different countries such as USA, India, Brazil, Spain, Germany, Thailand and Japan. NDM-1 is a metallo-β-lactamase (New Delhi metallo-β-lactamase) first isolated in India in 200 and now the isolates containing NDM-1 is rapidly growing in different parts of the world.